We want to discuss about defoamer chemistry in this article. After our launching the NS Defoamer, we do follow up with bubble management and silicone free defoamer to brief our reader from application perspective.
Besides the application perspective, we also want to talk about the defoamer from the chemistry point of view. This article will disclose some of the basic information how we formulate the silicone free NS Defoamer.
Even though we discuss about the defoamer chemistry but the actual defoamer selection is a kind of trial-and-error process.

The Defoamer Mechanism
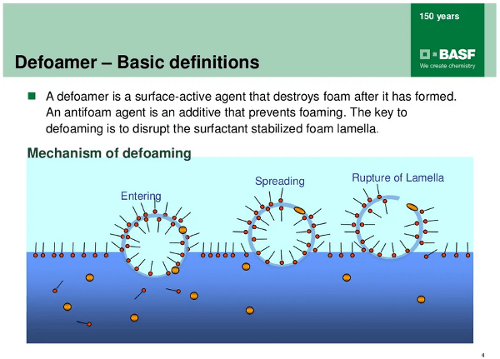
Foam is a trapped air in a liquid solution. The surfactant in the liquid solution which will lower down the surface tension meanwhile also increase the potential for generating bubble via agitation. While the bubble raise from the liquid toward the surface, a thin water film formed around the air. This is the formation of the foam.
The thin water film, lamella, becomes a dynamic barrier for the trapped air. It is so vibrant that 2 actions are happening in the foam simultaneously:
- The Drainage Effect.
- Protecting the trap air escape
The lamella becomes thinner during the drainage effect, the foam will burst if the lamella thickness reach 10nm. Under the drainage effect, the foam also conglomerate with densely pack with each another, at some level, the foam become “Dry Foam“. This means the foam formation reach the equilibrium process and will sustain for longer time.
Dry form comes in polyhedral formation instead of spherical formation.
Chemistry of Defoamer
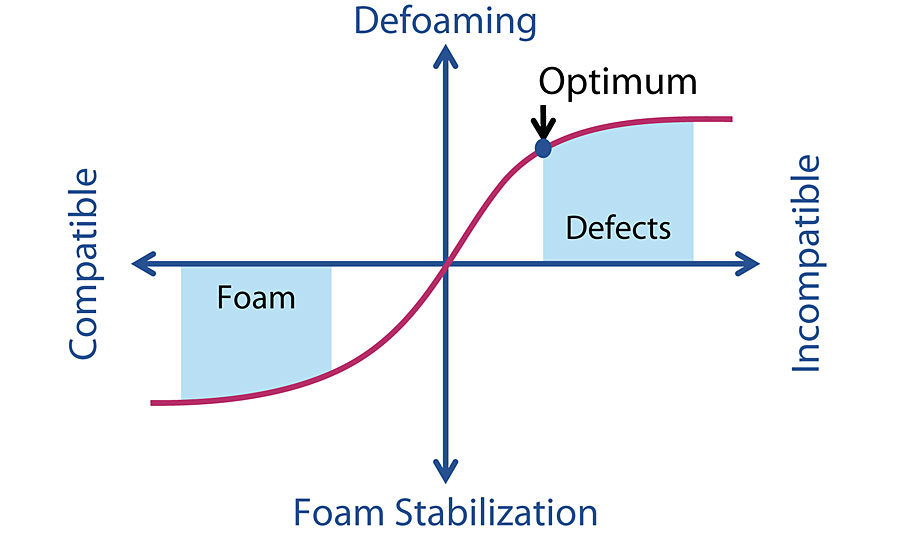
As we discuss earlier that the defoamer is changing the water film surface tension and cause the foam break. Hence, adding an distinct incompatible substance in the foam surface becomes our chemistry strategy for defoamer formulation.
Besides the incompatible characteristic, the defoamer substance also has to be insoluble in the liquid solution. This is because the surfactant comes in bi-polar characteristic, and the insoluble substance will pull the bi-polar chain out from the foam and causing the foam burst.
Now we are very clear what we actually looking for while formulate the defoamer. We have 3 type of chemistry plan, namely
- Mineral Oil base defoamer
- Silicone base defoamer
- Silicone-free polymeric defoamer
Currently, the market is fulled of Mineral oil and Silicone base defoamer. Silicone-free defoamer becomes the scarcity product and only served for limited user.
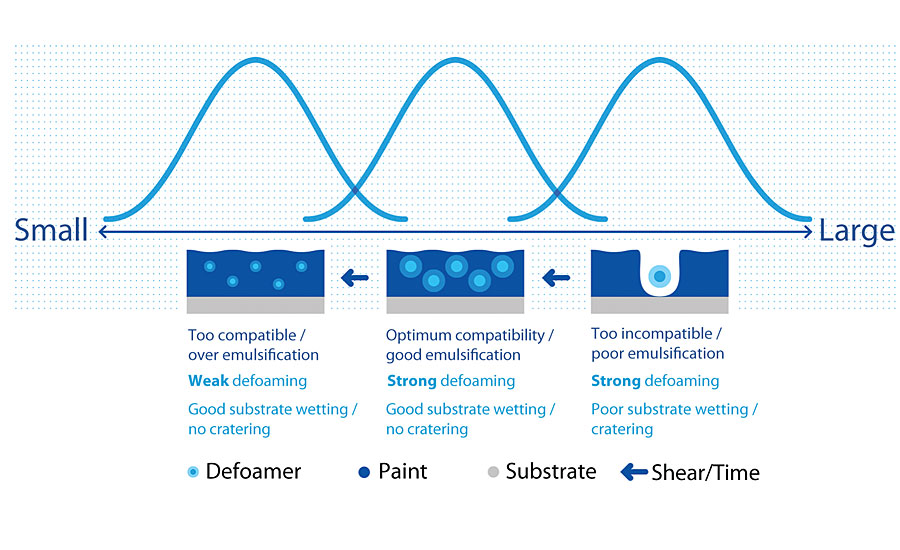
The user has to select silicone free defoamer if silicone contamination is not allowed in the process.
Defoamer Quality
Even though we already clearly aware the key defoamer chemistry, but practically tested still a must. With some simple observation and spray test, the field engineer should be able to tell if this defoamer is suitable.
- Defoamer stability. The defoamer should also maintain at homogeneous condition without separation.
- Foam breaking performance. The foam burst after get in touch with the defoamer.
- Residue on the substrate surface. The defoamer will rest on the substrate surface after the foam burst. The defoamer residue may cause defect on the substrate surface.
In DST, we may do some chemistry adjustment after we acquire the customer field test data. This is a tailor-made service whereby we could achieve a better defoamer product.
We reveal the defoamer chemistry to our reader so that there is a basic theory is supporting the formulation. This is a good moment if you are looking for silicone free defoamer in Malaysia . Feel free to contact DST about your needs.
